Low Iron Channel Glass: Achieving Exceptional Clarity through Technology and Cost Management
2025-06-30
Low iron channel glass, also known as ultra-clear or high-transparency channel glass, is an advanced, premium glass type recognized for its exceptional optical clarity, typically achieving over 91.5% visible light transmittance. Often dubbed the "Crystal Prince" within the glass industry, low iron channel glass is characterized by a crystal-clear appearance, elegance, and superior optical performance. Due to complex production methods, only a few global manufacturers—such as PPG (USA), Saint-Gobain (France), Pilkington (UK), Asahi Glass (Japan), and major Chinese producers like CSG, Taiwan Glass, and Xinyi Group—have mastered the technology. The combination of superior quality and higher cost has made low iron channel glass an emblem of architectural prestige.
Advantages of Low Iron Channel Glass:
Reduced Spontaneous Breakage Risk
Low iron glass contains significantly fewer impurities, such as nickel sulfide (NiS), thanks to meticulous quality control during production. This leads to a more homogeneous structure and notably reduces the risk of spontaneous breakage after tempering.Consistent and Neutral Color
With iron content ten times lower than regular glass, low iron glass reduces the absorption of green wavelengths in the visible spectrum. This ensures consistent color neutrality and unmatched optical clarity.High Transparency and Visibility
Offering more than 91.5% visible light transmission, low iron glass provides unmatched transparency and clarity. It vividly showcases displayed items, highlighting their true colors and details without distortion.Lower Ultraviolet Transmission
Low iron glass absorbs fewer UV rays compared to regular glass, making it ideal for environments sensitive to UV exposure, such as museums and galleries. Its properties effectively reduce UV damage, slowing fading and degradation of valuable artifacts, thus enhancing protection for displayed cultural relics.
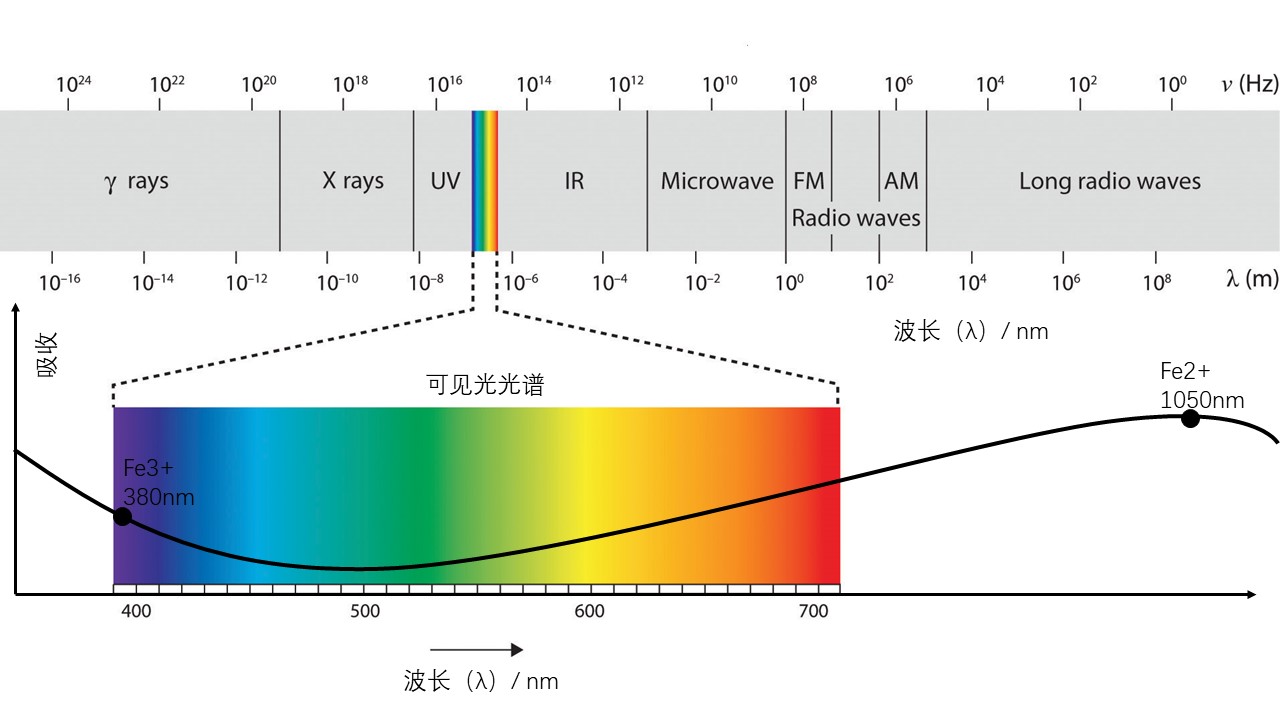
Why Does Ordinary Glass Appear Green?
Common glass typically has a greenish tint due to iron content naturally present in sand, its primary raw material.
Iron ions absorb blue and red wavelengths, thus allowing more green light to pass through.
Specifically, Fe²⁺ ions create a blue-green tint, while Fe³⁺ ions impart a yellow-green hue. Fe²⁺ has approximately ten times the coloring effect of Fe³⁺.
How Is Low Iron Channel Glass Produced?
Two common strategies improve glass transparency:
Reducing Iron Content
Using ultra-pure raw materials to significantly reduce the iron content in the glass composition.Oxidation of Iron Ions (Fe²⁺ to Fe³⁺)
Transforming Fe²⁺ ions into Fe³⁺ ions increases transparency by allowing more red wavelengths to pass through.
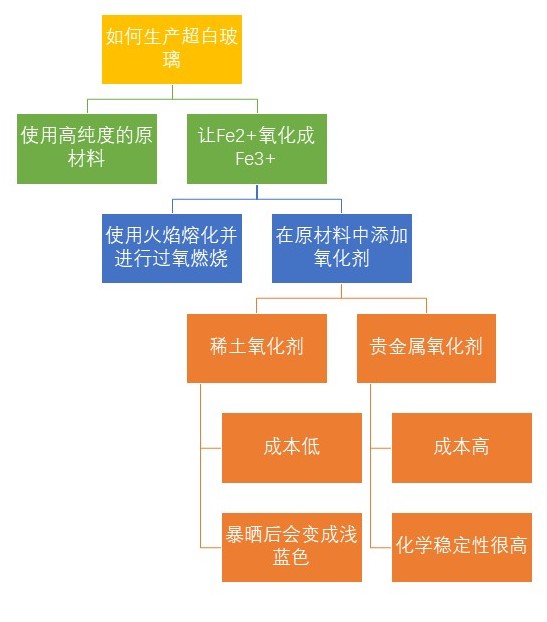
Two primary methods to oxidize Fe²⁺ to Fe³⁺ include:
Creating an Oxidizing Melting Environment
Employing fuel-based melting furnaces (instead of electrode melting) with an oxidizing atmosphere.Adding Oxidizing Agents
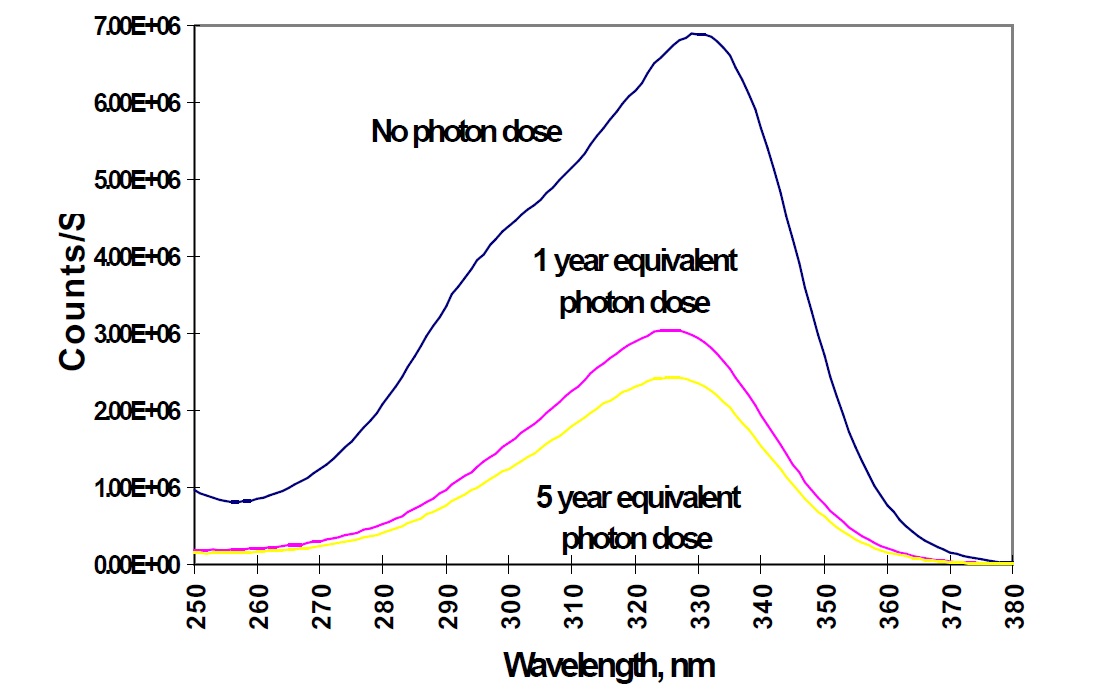
These oxidizers can be divided into two types:Rare Earth Oxidizers (Cerium Dioxide): CeO₂ effectively oxidizes Fe²⁺, but prolonged sun exposure can cause a "solarization" effect. This exposure gradually changes the glass color from light yellow to a bluish tint due to the oxidation of Ce³⁺ to Ce⁴⁺ and the reduction of Fe³⁺ to Fe²⁺.
Noble Metal Oxidizers: These oxidizers offer higher stability but at significantly higher costs.
Clearly, producing high-quality low iron channel glass requires sophisticated technology, careful raw material selection, and substantial investment.
Pitfalls: Inferior Low Iron Channel Glass Production
Lower-quality low iron glass commonly arises from cost-saving shortcuts:
Compromising Raw Materials
Some manufacturers use less expensive raw materials with higher iron content, thus compromising glass purity and introducing impurities, negatively affecting clarity.Excessive Use of Rare Earth Oxidizers
To mitigate higher iron content, some producers rely heavily on rare earth oxidizers like cerium dioxide. While effective initially, this method gives the glass a yellowish tint. To neutralize this tint, additives such as selenium, rubidium, or manganese are introduced, creating purple or pink hues. Instead of producing a true "colorless" glass, these glasses exhibit a "milky" or "foggy" appearance.


These production adjustments may suffice for lower-quality applications such as ordinary glassware. However, architectural applications—such as external facades or curtain walls—experience severe drawbacks, including rapid discoloration. Glass panels treated with excessive rare earth oxidizers frequently develop a purple hue and lose transparency within 1 to 2 years of exposure to sunlight, significantly diminishing their aesthetic appeal.
References:
[1] James E. Shelby. Glass Processing Course – Lecture 4: Color in Commercial Glasses.
[2] Zhang Weimin, Hou Yinglan. Coloring of Iron Oxide in Glass. Glass, Vol. 24, No. 6.
[3] Li Mei, Zhang Xiaowei, Liu Zhaogang, Hu Yanhong, Du Changshun. Applications of Cerium Oxide in Glass. Rare Earth, 2011, 32(3): 80-85.
[4] D.E. King, F.J. Pern, J.R. Pitts, C.E. Bingham, and A.W. Czanderna. "Optical Changes in Cerium-containing Glass as a Result of Accelerated Exposure Testing." 26th IEEE Photovoltaic Specialists Conference.